Permanganate ion can oxidize sulfite in basic solution according to the following equation. The relevant standard reduction potentials are 0.59 V for the manganese compound and 0.92 V for the sulfur compound. Determine the cell potential for the reaction at 298 K with the concentrations in the table. 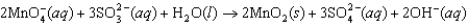 Species
Species
Concentration
MnO40.250 M
SO320.356 M
SO420.278 M
OH0.0222 M
Definitions:
Euro
The official currency used by 19 of the 27 European Union countries, also known by the acronym EUR.
Member Nations
Countries that are part of an international organization or agreement, and thus abide by its rules and contribute to its goals, such as the United Nations or European Union.
Volkswagen Beetle
A distinctive small car manufactured by Volkswagen, known for its iconic design and significant impact on automobile history.
Euros
The official currency of 19 out of the 27 European Union countries, which forms the eurozone.
Q7: Using the following data, determine the standard
Q7: Low-level nuclear waste from medical facilities generally
Q12: Calculate K for the following reaction, provided
Q18: Butanoic acid contributes to the rancid odor
Q30: Which of the following species is a
Q34: Identify two processes that convert a proton
Q67: What is true when a battery (voltaic
Q68: Write the equilibrium expression for the reaction
Q89: The diagram below represents a voltaic cell.
Q125: Inhibitors can operate by<br>I.decreasing the concentration of