In an experiment, 1.00 atm of N2(g) in a 10.0 L container at 25C was reacted under standard state conditions with a stoichiometric quantity of H2(g) to form ammonia: 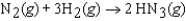 . What is the entropy change for the reaction?
. What is the entropy change for the reaction? 
Definitions:
Acid-Test Ratio
The Acid-Test Ratio, also known as the quick ratio, measures a company's ability to cover its short-term liabilities with its most liquid assets.
Q32: Which one of the following statements is
Q71: Two dipeptides can be produced from the
Q77: A person's body generates about 0.2 <font
Q103: Identify the weakest and strongest acids in
Q118: A solution with a pOH of 6.92
Q145: At 0 K, the entropy of a
Q150: Using the thermodynamic data below, determine the
Q154: The three-nucleotide sequence that codes for a
Q161: A barrel of oil produces about 5.9
Q166: Which statement is not correct? Sucrose _<br>A)is