Given the following data relevant to the combustion of ethanol, determine the free energy of formation for liquid ethanol, C2H5OH. 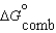 (C2H5OH, l) 930.7 kJ/mol
(C2H5OH, l) 930.7 kJ/mol 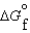 (CO2, g) 394.4 kJ/mol
(CO2, g) 394.4 kJ/mol 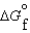 (H2O, g) 105.6 kJ/mol
(H2O, g) 105.6 kJ/mol
Definitions:
Free Radical Chlorine
A highly reactive species of chlorine that carries an unpaired electron, often involved in radical reactions.
Methane
A simple hydrocarbon with the formula CH4, representing the simplest alkane and a primary component of natural gas.
Regiochemistry
The study of the principles guiding the direction or position that new bonds form in a molecule during a chemical reaction.
Free Radical Halogenation
A chemical reaction where a halogen atom is introduced into an organic molecule by free radical mechanism, often used in the synthesis of halogenated compounds.
Q3: Which of the following can be mixed
Q49: Identify whether or not perturbations A-D will
Q49: In the following reaction in aqueous solution,
Q75: In a spontaneous process, which of the
Q77: Draw all of the isomers for butanol.
Q88: Which process converts a proton into a
Q89: Calculate the nuclear binding energy per nucleon
Q99: Which statement about a chemical reaction is
Q142: Which statement A-D, regarding the activity of
Q146: The entropy of a NaCl crystal is