The values of 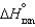 and
and 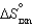 are shown for the following equilibrium reaction. What makes the equilibrium constant larger at low temperature?
are shown for the following equilibrium reaction. What makes the equilibrium constant larger at low temperature? 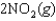
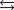
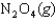 H 58.02 kJ/mol, S 176.5 J/mol K
H 58.02 kJ/mol, S 176.5 J/mol K
Definitions:
Member-Managed
Refers to a type of management structure in an LLC where all members participate in the decision-making processes.
Limited Liability Company
An enterprise configuration that amalgamates the individual taxation approach of sole proprietorships or partnerships with the capped liability advantages of a corporation.
Corporation
A legal entity that is separate and distinct from its owners, offering them limited liability and the ability to raise funds by selling shares.
Net Income
The profit remaining after all costs, expenses, and taxes have been subtracted from total revenue.
Q8: Purveyors of salts from the Dead Sea
Q17: In the smelting of iron from iron
Q24: Chemical equilibrium arises from the _ of
Q51: Which of the listed perturbations will change
Q52: The enthalpy of vaporization for toluene (C<sub>7</sub>H<sub>8</sub>)
Q71: Zinc-air batteries are actively being researched because
Q93: In the following reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="In
Q117: Which cell diagram is correct for this
Q126: An enzyme catalyst is effective because it
Q145: At 0 K, the entropy of a