Calculate the equilibrium constant at 500 K for the following reaction given the data below. 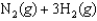
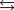
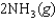 K c (298 K) 6.1 105
K c (298 K) 6.1 105 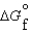 (NH3, g) 16.5 kJ/mol
(NH3, g) 16.5 kJ/mol 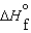 (NH3, g) 46.1 kJ/mol
(NH3, g) 46.1 kJ/mol
Definitions:
Skeptical Audiences
Groups or individuals who are inclined to doubt or question the validity of information presented to them.
Indirect Approach
A communication strategy that involves conveying a message in a roundabout way, rather than stating it directly, often used to soften the impact.
Hypothesis
A proposed explanation made on the basis of limited evidence as a starting point for further investigation.
Speculation
The act of forming theories or conjectures without firm evidence, often about financial markets or future events.
Q48: The half-life for a radioactive sample _<br>A)is
Q51: Oxidation is the _<br>A)gain of electrons.<br>B)loss of
Q53: Where in the periodic table do you
Q58: Under what conditions are the values of
Q61: An increase in the number of moles
Q84: In the following reaction, calculate Q, given
Q86: Addition of products to a chemical reaction
Q97: What units are used to measure the
Q102: Which statement is not correct? In hydrogen
Q175: If 1 mol of ice melts at