Noxious NO gas can form from N2 and O2 gases in automobile engines at high temperatures. If the value of the equilibrium constant, Kc, for this reaction at 25C is 1.95 1031, what is the value at 2,000C? 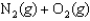
 Thermodynamic Properties of Nitrogen Monoxide
Thermodynamic Properties of Nitrogen Monoxide 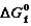
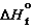 S87.6 kJ/mol 90.3 kJ/mol
S87.6 kJ/mol 90.3 kJ/mol
211 J/(mol K)
Definitions:
Efficiency
The ability to accomplish a task with a minimum expenditure of time and effort.
Curse of Knowledge
A cognitive bias that occurs when an individual, knowing something, finds it hard to imagine what it's like not to know it, affecting communication and teaching.
Computer Software
The collection of programs, procedures, and related data that instruct a computer what to do.
Employee Disengagement
A condition in which workers are emotionally disconnected from their work, leading to lower productivity, satisfaction, and morale.
Q32: Processes are always spontaneous when _ (H
Q70: If K for a reaction is large,
Q79: The diagram below represents a voltaic cell.
Q91: Nitrogen monoxide molecules can react to form
Q105: Which of the following processes will lead
Q106: Which sodium halide salt, if any, would
Q117: Which of the following will have the
Q160: An electrochemical cell is constructed with a
Q162: Cobalt-60 decays to nickel-60. What particle is
Q165: The entropy change in the surroundings (<font