Calculate the equilibrium constant at 500 K for the following reaction given the data below:
N2(g) 3H2(g) 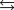 2NH3(g)
2NH3(g)
Kc (298 K) 6.1 105 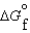 (NH3, g) 16.5 kJ/mol
(NH3, g) 16.5 kJ/mol 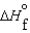 (NH3, g) 46.1 kJ/mol
(NH3, g) 46.1 kJ/mol
Definitions:
Mislaid Property
Items that are intentionally placed somewhere by their owner and then forgotten, distinguishing from lost or abandoned property.
Lost Property
Items that have been unintentionally left by their owner at a place where they cannot be found.
Backpack
A portable container designed to be carried on one's back, typically secured with two straps over the shoulders.
Intangible Property
Assets that have no physical presence but have value, such as copyrights, trademarks, and patents.
Q19: Radiation seed therapy is a common method
Q51: The rate of popcorn popping at different
Q69: Smog created by the interaction of sunlight
Q76: If the reaction quotient Q is equal
Q81: Which statement about this concentration cell is
Q83: Halfway to the equivalence point in a
Q97: What reaction occurs as a hydrochloric acid
Q115: Determine the molar concentration of Fe<sup>3</sup><font face="symbol"><sup></sup></font>(aq)
Q135: The symbol <font face="symbol"></font><font face="symbol"></font> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg"
Q165: The entropy change in the surroundings (<font