The values of 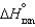 and
and 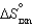 are shown for the following equilibrium reaction. What makes the equilibrium constant larger at low temperature?
are shown for the following equilibrium reaction. What makes the equilibrium constant larger at low temperature? 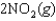
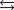
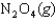 H 58.02 kJ/mol, S 176.5 J/mol K
H 58.02 kJ/mol, S 176.5 J/mol K
Definitions:
Oedipus Complex
A psychoanalytic theory that describes a child's feelings of desire for the opposite-sex parent and jealousy and competitiveness with the same-sex parent.
Developmental Process
The series of changes or growth that an organism undergoes over its lifespan, from conception to death.
False Memories
An occurrence in which an individual remembers an event inaccurately or remembers something that never actually took place.
Achievement Motivation
The drive or intrinsic motivation that leads individuals to pursue and attain goals.
Q31: Indicate which one of the following reactions
Q33: If the rate of formation of ammonia
Q41: Considering the tabulated values for the thermodynamic
Q53: Determine the standard entropy of N<sub>2</sub>(g) given
Q59: For the rate law Rate <font face="symbol"></font>
Q78: Which of the following compounds would not
Q81: A chemical equilibrium 2<sub> </sub>A <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg"
Q114: In the following reaction, which species is
Q130: Jane can accept that <font face="symbol"></font>G<font face="symbol"></font>
Q154: During a spontaneous chemical reaction, it is