Calculate the equilibrium constant at 500 K for the following reaction given the data below. 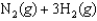
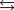
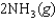 K c (298 K) 6.1 105
K c (298 K) 6.1 105 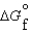 (NH3, g) 16.5 kJ/mol
(NH3, g) 16.5 kJ/mol 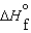 (NH3, g) 46.1 kJ/mol
(NH3, g) 46.1 kJ/mol
Definitions:
Tax Returns
Official documents filed with tax authorities that report income, expenses, and other pertinent tax information.
Q1: Identify the dominant species that determine the
Q10: One mole of solid ammonium carbamate (NH<sub>4</sub>CO<sub>2</sub>NH<sub>2</sub>)
Q72: Boric acid frequently is used as an
Q76: Calculate the nuclear binding energy per nucleon
Q84: To simulate the pH of blood, which
Q85: Suppression of the solubility of one ion
Q105: The unit of current, ampere (A), is
Q139: If a reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="If a
Q159: The half-life of a radioactive isotope is
Q161: The thermite reaction, shown below, is very