Noxious NO gas can form from N2 and O2 gases in automobile engines at high temperatures. If the value of the equilibrium constant, Kc, for this reaction at 25C is 1.95 1031, what is the value at 2,000C? 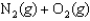
 Thermodynamic Properties of Nitrogen Monoxide
Thermodynamic Properties of Nitrogen Monoxide 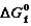
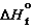 S87.6 kJ/mol 90.3 kJ/mol
S87.6 kJ/mol 90.3 kJ/mol
211 J/(mol K)
Definitions:
Structural Functionalism
A sociological approach that sees society as a complex system whose parts work together to promote solidarity and stability.
Stability
The state of being steady, consistent, and relatively unchanged, often regarded as a desirable condition in various contexts such as social, economic, and ecological systems.
Structural Functionalism
This theory posits that society functions as an intricate system, with its components cooperating to maintain cohesion and steadiness.
Social Feature
An aspect or characteristic of society that influences human behaviors, interactions, and relationships.
Q6: Which statement about an equilibrium constant, K,
Q16: In each of the following, the stronger
Q30: When a molecule of ethylenediamine replaces two
Q37: In evaluating the pH of an aqueous
Q71: You have a summer job as an
Q102: Lead pipes were used at one time
Q119: When a solution of DNA in water
Q133: Carbon-14 measurements on the linen wrappings from
Q164: For a particular hypothetical reaction, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg"
Q164: The argon-40 method of radioisotope dating was