Which statement about the reaction below, where en ethylenediamine (H2NCH2CH2NH2) , is not correct? 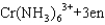
Definitions:
Incremental Value
The additional or extra value generated by making a specific business decision, considered when evaluating multiple options.
Earnings per Share
A financial ratio indicating the portion of a company's profit allocated to each outstanding share of common stock, serving as an indicator of the company’s profitability.
Merger Premium
The additional cost or amount by which a company's purchase price exceeds the pre-merger valuation of the target company.
Stand-Alone Value
The intrinsic worth of an investment or project if undertaken independently, without considering synergies or external factors.
Q5: Determine the entropy change for the reaction
Q19: What happens to the equilibrium between NO<sub>2</sub>(g)
Q40: Given the reaction below, which of the
Q52: Identify the weakest and strongest acids in
Q89: The diagram below represents a voltaic cell.
Q95: A solution of the weak acid HF
Q107: Strontium-90 is most likely to decay by
Q114: Tritium (<sup>3</sup>H) is used in glowing "EXIT"
Q131: In a Geiger counter, which is used
Q161: A barrel of oil produces about 5.9