In an experiment, 1.00 atm of N2(g) in a 10.0 L container at 25C was reacted under standard state conditions with a stoichiometric quantity of H2(g) to form ammonia: 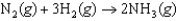 .
.
What is the entropy change for the reaction? 
Definitions:
Value Chain Optimization
The process of increasing the efficiency and effectiveness of a value chain, involving activities that add value to a product or service from its conception to delivery to the consumer.
Logistics
The detailed coordination and implementation of complex operations, often referring to the movement and storage of goods from point of origin to point of consumption.
Electronic Data Interchange
A system that allows the transfer of information between organizations in a structured format, facilitating automatic processing and reducing manual errors.
ERP Systems
Integrated software platforms used by organizations to manage and automate many back office functions related to technology, services, and human resources.
Q6: Which of the following is/are true for
Q7: Low-level nuclear waste from medical facilities generally
Q40: What are the signs of <font face="symbol"></font>H
Q41: A solution of sulfuric acid (H<sub>2</sub>SO<sub>4</sub>, 25.00
Q60: How much work can be done using
Q65: An important technique used to monitor cell
Q72: What type of motion is depicted in
Q124: Before class, students were distributed throughout a
Q130: Jane can accept that <font face="symbol">��</font>G<font face="symbol"></font>
Q131: Using the thermodynamic data below, determine the