Calculate the equilibrium constant at 500 K for the following reaction given the data below:
N2(g) 3H2(g) 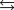 2NH3(g)
2NH3(g)
Kc (298 K) 6.1 105 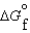 (NH3, g) 16.5 kJ/mol
(NH3, g) 16.5 kJ/mol 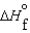 (NH3, g) 46.1 kJ/mol
(NH3, g) 46.1 kJ/mol
Definitions:
Annual Amortization Expense
The portion of the cost of an intangible asset that is expensed through an organization's income statement each year over the asset's useful life.
Consolidated Tax Return
A tax return that combines the tax liability of all subsidiary companies with that of a parent company, treating them as one entity for tax purposes.
Domestic Subsidiaries
Subsidiaries located in the same country as the parent company, operating under the laws and regulations of that country.
Foreign Subsidiaries
Companies that are owned or controlled by another corporation (the parent company) and are located in a country different from where the parent company operates.
Q13: Calculate the K<sub>sp</sub> for Li<sub>3</sub>PO<sub>4 </sub>if at
Q23: Given the following titration curves, provide a
Q42: Which of the following species is a
Q51: Which of the listed perturbations will change
Q83: Uranium-238 ( <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="Uranium-238 (
Q90: Solutions of each of the hypothetical bases
Q92: Given the following data for the reaction
Q108: Define the term Lewis acid and provide
Q126: What is the pH of a solution
Q159: Chromium often is electroplated on other metals