At what point in the following titration curve for a weak acid being titrated with a strong base is the pH equal to the pKa of the acid? The x-axis scale goes from 0.0 mL to 20.0 mL. The sharp rise is at 10.0 mL. 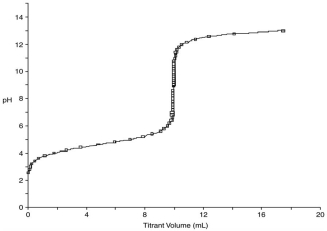
Definitions:
Leadership Inversion
A situation where the traditional roles of leaders and followers are reversed, emphasizing the empowerment and decision-making capabilities of lower-level employees.
Visibility
The degree to which an individual's activities are noticed, recognized, and regarded by others within an organization or society.
Organizational Politics
The use of power and social networking within an organization to achieve changes that benefit the individual or the organization.
The Woods
A large area covered chiefly with trees and undergrowth.
Q35: Why is iodine-131 used effectively to treat
Q56: A proposed mechanism for the decomposition of
Q64: Which, if any, of statements A-D is
Q65: Acid-base indicators need to have very intense
Q81: How would you calculate K<sub>b</sub> for the
Q82: Which of the processes A-D will lead
Q90: In a simple equilibrium A <font face="symbol"></font>
Q142: Boltzmann derived the relationship S <font face="symbol"></font>
Q142: In a catalyzed reaction, the _ of
Q153: The standard molar entropy of silver chloride