Calculate the equilibrium concentration of Zn2(aq) in a solution that is 0.0125 M Zn(NO3) 2 and 0.600 M NH3.
Given: Zn2(aq) 4NH3(aq) 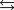 Zn(NH3) 42(aq) Kf 2.9 109.
Zn(NH3) 42(aq) Kf 2.9 109.
Definitions:
Breach of Contract
The failure to perform any term of a contract, written or oral, without a legitimate legal excuse, leading to legal actions for remedies.
Statute of Frauds
A legal principle requiring certain contracts to be in writing and signed by the party to be charged, to prevent fraud and perjuries.
Antiassignment Clause
A provision in a contract that restricts the transfer of rights or obligations to another party without prior approval.
Operation of Law
Changes in rights or duties that take place automatically under the law, without any action by the parties involved.
Q14: The stronger the acid, _<br>A)the stronger its
Q36: Which expression corresponds to the equilibrium constant
Q67: Sometimes liquid ammonia, NH<sub>3</sub>, is used as
Q70: Which of the following statements must be
Q89: A Lewis base is _<br>A)an electron-pair acceptor.<br>B)an
Q97: What units are used to measure the
Q102: A voltaic cell is constructed based on
Q105: Which of the following processes will lead
Q110: Which of the following is the equilibrium
Q110: A solution with a pH of 9.50