Given the following reactions and associated equilibrium constants 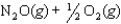
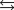 2NO(g) K1 1.70 1013
2NO(g) K1 1.70 1013 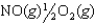
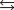 NO2(g)
NO2(g)
K2 6.83 106
What is the equilibrium constant for the following reaction?
2N2O(g) 3O2(g) 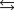 4NO2(g) Knew ?
4NO2(g) Knew ?
Definitions:
Population Trends
Patterns of change in the size, composition, and distribution of human populations over time.
Dwelling Type
Dwelling type refers to the classification of residential units based on their structure, design, and functionality, such as apartments, houses, or townhomes.
State of Nature
The actual condition or reality of a situation which decision makers do not control and must consider in their planning.
Decision Nodes
Points within a decision tree at which choices are available, representing different paths that can be taken.
Q17: Lead poisoning used to be treated by
Q18: If the rate of formation of ammonia
Q36: An atom in a particular unit cell
Q40: What are the signs of <font face="symbol"></font>H
Q56: A proposed mechanism for the decomposition of
Q65: If for a given chemical reaction at
Q91: Nitrogen monoxide molecules can react to form
Q110: A 75 mg sample of a natural
Q120: Which of the following must be true
Q176: The standard entropy of nitrogen gas in