Sulfur dioxide, gaseous water, and oxygen gas react to prepare sulfuric acid in the following unbalanced equation. 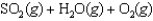
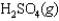 Under equilibrium conditions, the partial pressures of the components are PSO2 0.1 atm, PH2O 0.05 atm, PO2 0.25 atm, PH2SO4 2.75 atm.
Under equilibrium conditions, the partial pressures of the components are PSO2 0.1 atm, PH2O 0.05 atm, PO2 0.25 atm, PH2SO4 2.75 atm.
What is the value of the equilibrium constant, Kp, for the reaction?
Definitions:
Circumference
The linear distance around the edge of a circle or spherical object.
James's Claim
A reference to a specific assertion or theory proposed by psychologist William James, often pertaining to his contributions to psychology, such as the James-Lange theory of emotion.
Brain Development
The process of growth and change in the brain's structure and function over the course of a lifetime, particularly significant in early childhood.
Myelination
The process of forming a myelin sheath around nerves, improving the transmission speed of nerve impulses.
Q2: As the pH increases, the solubility of
Q7: Consider a closed container containing a 1
Q25: A chemical equilibrium <sub> </sub><sub> </sub>A <img
Q27: Which solution, if either, would create the
Q51: What is the solubility of barium sulfate
Q92: A Lewis acid is any species capable
Q95: Which of the following occurs when products
Q107: Bronze that is composed of 10% tin
Q114: In the following reaction, which species is
Q132: Which of the following ionic compounds would