Water can decompose at an elevated temperature to give hydrogen and oxygen according to the equation below. At a particular temperature, the partial pressures of H2O, H2, and O2 are 0.055 atm, 0.0065 atm, and 0.0045 atm, respectively, at equilibrium. What is the value of the equilibrium constant, KP, for this reaction at this temperature? 2H2O(g) 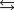 2H2(g) O2(g)
2H2(g) O2(g)
Definitions:
Systems Design
The process of defining the architecture, components, interfaces, and data for a system to satisfy specified requirements.
Income Statement
A financial report that shows a company's revenues, expenses, and profits over a specific accounting period.
E-Commerce
The use of the Internet for performing business transactions.
Subsidiary Ledgers
Detailed records that support summary-level data contained in the general ledger, such as accounts receivable and accounts payable ledgers.
Q15: How many nearest neighbor atoms are there
Q29: Hydrochloric acid (HCl) reacts with sodium hydroxide
Q30: A rock salt structure has smaller ions
Q31: In the diagram below, one beaker contains
Q34: The energy profiles for four different reactions
Q39: Suppose <font face="symbol"></font>G<font face="symbol"></font> is -2.0 kJ/mol
Q87: The dissolution of ammonium nitrate in water
Q89: Collision theory assumes that the rate of
Q96: Which of the following processes is/are reversible
Q114: For the reaction A <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="For