Given the following reactions and associated equilibrium constants 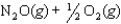
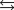 2NO(g) K1 1.70 1013
2NO(g) K1 1.70 1013 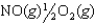
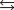 NO2(g)
NO2(g)
K2 6.83 106
What is the equilibrium constant for the following reaction?
2N2O(g) 3O2(g) 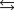 4NO2(g) Knew ?
4NO2(g) Knew ?
Definitions:
Contract
A contract recognized by law, involving at least two parties, each with obligations to the other.
Principal
The original amount of money invested or loaned, before interest, or the primary party engaged in a contract or transaction.
Authority
The legal power or right given to an individual or entity to make decisions, and enforce obedience.
Agents
Persons authorized to act on behalf of another, known as the principal, in legal or business matters.
Q22: Magnesium chloride is often used to melt
Q34: When an acetic acid solution is titrated
Q47: If <font face="symbol"></font><font face="symbol"></font> <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="If
Q54: The reaction A(g) <font face="symbol"></font> B(g) <img
Q77: Would you expect the linear allotrope of
Q78: Gaseous hydrogen and iodine can be used
Q79: Which statement regarding osmotic pressure is not
Q85: Indicate which aqueous solution has the slowest
Q98: Which of the following is true for
Q99: An ice cube at 0<font face="symbol"></font>C melts