Solid mercury(II) oxide decomposes when heated to produce liquid mercury and oxygen. 2HgO(s) 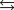 2Hg(l) O2(g)
2Hg(l) O2(g)
An amount of mercury(II) oxide is placed in a vessel at a particular temperature and allowed to reach equilibrium. How could the amount of liquid mercury in the vessel be increased?
I. adding more mercury(II) oxide
II. removing some oxygen
III. increasing the volume of the vessel
Definitions:
Statute of Frauds
is a legal principle that requires certain contracts to be in writing and signed by the party to be charged, in order to be enforceable.
Statute of Frauds
The Statute of Frauds is a legal concept that requires certain types of contracts to be in writing and signed by the party to be charged, in order to be legally enforceable.
Statute of Frauds
A legal principle requiring certain types of contracts to be written and signed to be enforceable, aiming to prevent fraud and misunderstandings.
UCC
The full spectrum of commercial dealings in the United States is under the regulation of the laws within the Uniform Commercial Code.
Q13: Calculate the K<sub>sp</sub> for Li<sub>3</sub>PO<sub>4 </sub>if at
Q32: Processes are always spontaneous when _ (H
Q58: Under what conditions are the values of
Q72: Research with biochemical systems commonly requires buffers
Q86: Write out the five steps of the
Q103: Indicate which one of the following reactions
Q110: Which of the following is the equilibrium
Q111: A sketch of the free energy for
Q113: Which arrangement below orders the cations from
Q124: Which of the following solvents will involve