Multiple Choice
In the Reaction, Initial, Change, Equilibrium (RICE) table started for calculating equilibrium concentrations of the reaction shown, the terms in the "change" row are ________ 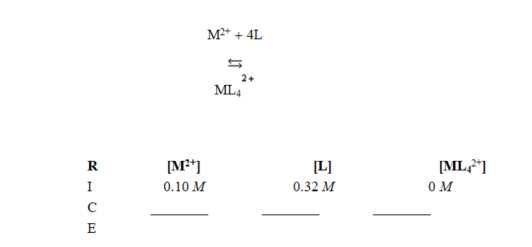
Definitions:
Related Questions
Q10: Before class, students were seated at three
Q11: The cubic closest-packed crystal structure has a
Q17: In a first-order reaction, the initial concentration
Q22: Which statement does not describe a simple
Q38: If the rate of the reaction: <img
Q48: The pK<sub>a</sub> of a weak acid was
Q50: Compare the packing efficiency of face-centered cubic
Q61: Which one of the following salts forms
Q76: If the reaction quotient Q is equal
Q109: What is the value of the equilibrium