The equilibrium constant for the reaction below is 17.5.
S(s) 3O2(g) 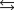 2SO3(g)
2SO3(g)
What is the value of the equilibrium constant for the following reaction?
6SO3(g) 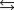 3S(s) 9O2(g)
3S(s) 9O2(g)
Definitions:
Self-Serving Principles
Guidelines or beliefs that are primarily aimed at benefiting oneself, often at the expense of others.
Concern for Others
A trait or attitude that reflects a person's care, empathy, and consideration for the feelings and needs of other people.
Situational-Effect Principles
The concept that the outcomes or behaviors in a given situation are influenced by the unique combination of elements present in that situation.
Concern-For-Others Principles
The concern-for-others principles prioritize empathy, care, and the welfare of others in decision-making and interpersonal interactions, fostering a supportive and compassionate environment.
Q39: The face-centered cubic unit cell has _
Q45: The sulfide ion, S<sup>2</sup><font face="symbol"><sup></sup></font>, reacts with
Q51: Determine the value of <font face="symbol"></font>G<font face="symbol"></font>
Q54: The reaction A(g) <font face="symbol"></font> B(g) <img
Q60: If the reaction quotient Q has a
Q64: What information is provided by the van
Q68: Write the equilibrium expression for the reaction
Q74: Sulfur dioxide emitted in the burning of
Q81: The rate of popcorn popping at different
Q90: Solutions of each of the hypothetical bases