Octane can undergo combustion to produce carbon dioxide and water and release tremendous amounts of energy. The balanced chemical equation for this reaction is shown below. How could the rate of this reaction be expressed correctly in terms of the rate at which the concentration of a reactant or product changes? 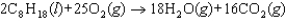
Definitions:
Organic Product
Organic product refers to any compound containing carbon atoms that is produced as a result of an organic chemical reaction.
Primary Alcohols
Organic compounds featuring a hydroxyl group (-OH) attached to a primary carbon atom, often reactive and used in the production of various chemicals and solvents.
Carboxylic Acids
Organic compounds characterized by the presence of at least one carboxyl group (-COOH), which includes substances like acetic acid and butyric acid.
Lithium Aluminum Hydride
A powerful reducing agent used in organic synthesis to reduce esters, carboxylic acids, and amides to alcohols.
Q35: A qualitative interpretation of the effect of
Q39: The first disinfectant used by Joseph Lister
Q68: Three acids found in foods are lactic
Q92: For an equilibrium reaction with K <font
Q110: A 75 mg sample of a natural
Q117: Why does a pure metal not crystallize
Q118: Which point in the reaction profile below
Q120: If half of the tetrahedral holes in
Q138: Determine the molal concentration of a sugar
Q142: Boltzmann derived the relationship S <font face="symbol"></font>