Using the reaction shown below, which statement is correct? 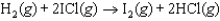
Definitions:
Fair Value
The capital retrieved from trading an asset or the expenditure for reallocating a liability in a well-arranged market transaction at the point of assessing value.
Carry Value
The original purchase cost of an asset adjusted for depreciation, amortization, or impairment costs on the financial statements.
Joint Operation
A business arrangement where two or more parties share control and management of a project or business.
Joint Venture
A business arrangement where two or more parties agree to pool their resources for the purpose of accomplishing a specific task or business activity.
Q13: As an analog of graphite, a material
Q20: The closest-packing of spheres (such as oranges,
Q50: Arrange the following compounds in order of
Q51: What is the solubility of barium sulfate
Q83: Which of the following will have the
Q85: Consider the three unit cells below for
Q90: The term colligative refers to properties that
Q94: The vapor pressure of a liquid increases
Q100: Cylinders of NO gas may contain small
Q152: Nitric oxide and nitrogen dioxide are found