Multiple Choice
The following figure shows Arrhenius plots for four different reactions. Which reaction has the lowest activation energy? 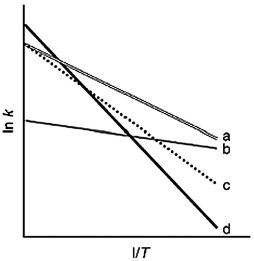
Definitions:
Related Questions
Q4: The phase diagram for carbon dioxide is
Q14: The equilibrium constants for the two reactions
Q19: What happens to the equilibrium between NO<sub>2</sub>(g)
Q31: Which of the following compounds will have
Q57: What does the line indicated by the
Q61: Which of the following is the best
Q100: The hydronium ion concentration of a dilute
Q102: Which unit cell contains the least atoms?<br>A)fcc<br>B)bcc<br>C)sc<br>D)both
Q105: Predict the relationship between the lattice energies
Q108: What is the concentration of [OH<font face="symbol"><sup></sup></font>]