The mechanism for the reaction 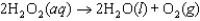 in the presence of I(aq) is proposed to be:
in the presence of I(aq) is proposed to be:
Step 1: 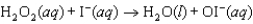 (slow)
(slow)
Step 2: 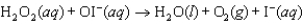 (fast)
(fast)
Identify a) any intermediates, b) any catalysts, and c) the rate law for the reaction.
Definitions:
Double-Declining-Balance
An accelerated method of depreciation which doubles the straight-line depreciation rate, applying it to the declining balance of the asset's cost.
Depreciation Journal Entry
An accounting record that reflects the allocation of the cost of a tangible asset over its useful life.
Depreciable Cost
The total cost of an asset that is subject to depreciation over its useful life, excluding salvage value.
Double-Declining-Balance Rate
A method of accelerated depreciation which doubles the rate at which an asset’s book value is depreciated compared to straight-line depreciation.
Q4: A proposed mechanism for the decomposition of
Q51: What is the pH of a 0.055
Q56: How many nearest neighbor atoms are there
Q62: Identify the following statement as true or
Q73: Which one of the ionic compounds below
Q101: Consider the reaction of nitrogen and hydrogen
Q107: A triatomic molecule with a bond angle
Q118: A solution with a pOH of 6.92
Q138: Why do the strengths of dispersion interactions
Q149: Which of the following requires the smallest