Calculate the lattice energy of sodium fluoride from the following data: Ionization energy of Na: 496 kJ/mol
Electron affinity of F: 328 kJ/mol
Energy to vaporize Na: 108 kJ/mol
F2 bond energy: 160 kJ/mol
Energy change for the reaction: 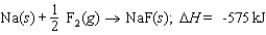
Definitions:
Bottom Line
The final result or the most important point, especially in the context of business referring to net income or profit.
Retention Success
A measure of an organization's ability to keep employees over time, reflecting effective HR policies and practices.
Employee Moral
The overall emotional and mental condition of staff members regarding their workplace, often influenced by leadership, work conditions, and job satisfaction.
Tracking System
A technology or method used to monitor the movement, progress, or status of items, individuals, or processes.
Q17: Water forms a concave meniscus in a
Q17: Methylamine (CH<sub>3</sub>NH<sub>2</sub>) is a weakly basic compound.
Q28: For the rate law Rate <font face="symbol"></font>
Q40: Arrange the first four halogens in order
Q62: Define a conjugate acid<font face="symbol"></font>base pair and
Q76: Write the chemical reaction using skeleton structures
Q119: In a(n) _ step of a reaction
Q123: What structural characteristics must a molecule have
Q132: What is the molecular geometry of the
Q140: Describe how the data obtained by a