Calculate the lattice energy of magnesium chloride from the following data: First ionization energy of Mg: 738 kJ/mol
Second ionization energy of Mg: 1,451 kJ/mol
Electron affinity of Cl: 349 kJ/mol
Energy to vaporize Mg: 147.1 kJ/mol
Cl2 bond energy: 243 kJ/mol
Energy change for the reaction: 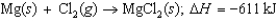
Definitions:
Motivation
The process that initiates, guides, and maintains goal-oriented behaviors.
Frustrations
Feelings of dissatisfaction or annoyance arising from obstacles hindering the achievement of a goal or desire.
Planning Fallacy
The tendency to underestimate the time, costs, and risks of future actions, while overestimating the benefits, leading to overly optimistic project timelines and outcomes.
Hierarchy Of Goals
A system of organizing objectives that range from high-level, long-term goals to lower-level, short-term actions, enabling structured pursuit of ambitions.
Q28: For the rate law Rate <font face="symbol"></font>
Q34: Consider the phase diagram for a substance
Q35: Rank the following ionic compounds in order
Q60: Identify which of the following alkanes has
Q60: The half-life (t<sub>1/2</sub>) of a first-order reaction
Q76: What is the molality of a solution
Q113: The density of water decreases as it
Q126: If the rate of formation of ammonia
Q126: In the following Born-Haber cycle for the
Q138: The photochemical decomposition of chlorofluorocarbons <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg"