Determine the energy change for the reaction 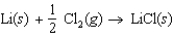 from the following data: Lattice energy of LiCl 861 kJ/mol
from the following data: Lattice energy of LiCl 861 kJ/mol
Energy to vaporize Li 159 kJ/mol
Ionization energy of Li 520 kJ/mol
Cl2 bond energy: 240 kJ/mol
Electron affinity of Cl: 349 kJ/mol
Definitions:
Power Relations
The dynamics and structures of authority and influence among individuals, groups, and institutions within a society.
Unequal Power
A situation or relationship where power is distributed unevenly, leading to dominance by one group or individual over another.
Researchers
Individuals who conduct detailed studies and systematic investigations to discover or establish facts and reach new conclusions.
Theoretical Perspectives
Different lenses or frameworks through which sociologists view, analyze, and interpret social phenomena and human behaviors.
Q3: The following Lewis symbol corresponds to which
Q26: Using the energy-level diagram for valence orbitals
Q29: What type of hole is depicted below?
Q36: An atom in a particular unit cell
Q47: Which of the following is true for
Q50: The pH of an aqueous sodium fluoride
Q96: Identify the weakest and strongest acids in
Q122: Rank the following ionic compounds based on
Q129: For each of the following pairs of
Q186: Describe the valence bond picture of bonding