Multiple Choice
Calculate the lattice energy of sodium fluoride from the following data: Ionization energy of Na: 496 kJ/mol
Electron affinity of F: 328 kJ/mol
Energy to vaporize Na: 108 kJ/mol
F2 bond energy: 160 kJ/mol
Energy change for the reaction: 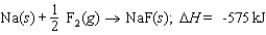
Definitions:
Related Questions
Q5: Which one of the following substances would
Q21: Effects that stabilize the conjugate base of
Q53: Based on their boiling points, which of
Q55: NO<sub>2</sub> concentrations during photochemical smog events often
Q71: Ethylene glycol is used commonly as an
Q99: The alpha form of polonium (Po) has
Q105: What is the pH of a 0.500
Q109: What is the hydronium ion concentration of
Q123: Quartz, a form of SiO<sub>2</sub>, which contains
Q140: The local molecular geometry and the hybridization