Multiple Choice
Determine the energy change for the reaction 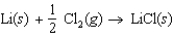 from the following data: Lattice energy of LiCl 861 kJ/mol
from the following data: Lattice energy of LiCl 861 kJ/mol
Energy to vaporize Li 159 kJ/mol
Ionization energy of Li 520 kJ/mol
Cl2 bond energy: 240 kJ/mol
Electron affinity of Cl: 349 kJ/mol
Definitions:
Related Questions
Q2: Which of the following diagrams shows the
Q12: Would water rise to the same height
Q15: Which one of the following molecules has
Q20: When can an x be ignored in
Q47: Of the following molecules (O<sub>3</sub>, SCl<sub>2</sub>, SO<sub>2</sub>,
Q61: Which one of the following salts forms
Q77: Explain why some salts produce neutral solutions
Q92: What is the pH of a 0.35
Q113: Which arrangement below orders the cations from
Q132: What is the molecular geometry of the