The carbon dioxide pressure in a bottle of champagne is about 6 atm. At this pressure about 0.45 g of carbon dioxide dissolves in 100 mL of champagne. How much carbon dioxide remains dissolved in 100 mL after the bottle is opened? The partial pressure of carbon dioxide then is 6.0 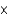 104 atm.
104 atm.
Definitions:
Q2: Which of the following aqueous solutions will
Q3: Which alkane compound has the highest vapor
Q8: Which one of the following molecules violates
Q17: Which bond is the most polar?<br>A)carbon-oxygen<br>B)carbon-sulfur<br>C)carbon-nitrogen<br>D)carbon-silicon<br>E)carbon-fluorine
Q39: CH<sub>2</sub>F<sub>2</sub> has a dipole moment of 1.93
Q61: The relative energies (strengths) of the intermolecular
Q68: In the cubic closest-packed structure of sodium
Q100: What is the valence electron molecular orbital
Q124: Which of the following solvents will involve
Q133: Write definitions for the terms miscible and