Oxygen has two common molecular anions: peroxide (O22) and superoxide (O2) . Use the MO energy level diagram below to identify which one of the following statements is not correct. These molecular orbitals are formed from the 2s and 2p atomic orbitals. 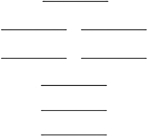
Definitions:
Sales Representatives
Individuals who represent a company by selling its products or services to customers, often working to meet sales targets and maintain customer relationships.
Democratic Leader
A leadership style where decisions are made based on the input of team members, promoting participation and equality among the group.
Employee Participation
Refers to the involvement of employees in decision-making processes and contributing to the improvement of organizational practices.
Tasks Over People
A management approach that prioritizes work and tasks achievement over attending to the well-being and needs of employees.
Q37: Why does HI boil at a higher
Q45: `If cesium, which has a work function
Q47: What is the kinetic energy of the
Q69: Select which of the following repulsive interactions
Q83: Which of the following monomers is used
Q87: What is the pressure of NO gas
Q91: When two liquids mix completely in all
Q109: Which of the following, based on formal
Q116: How many lone-pair electrons are on each
Q135: How many valence electrons are there in