Which one of the following molecules is paramagnetic? These molecules are described by the MO energy diagram below. These molecular orbitals are formed from the 2s and 2p atomic orbitals. 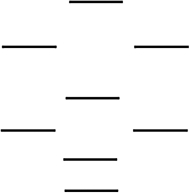
Definitions:
Private Value
The individual valuation of a good or service to a particular consumer or producer, not taking into account external effects or social welfare.
External Benefits
Positive effects of a product or service on individuals or entities who are not directly involved in the transaction or production of the product or service.
External Benefit
A positive effect or advantage that results from a product or service's use, affecting individuals or entities who are not directly involved in the transaction.
Positive Externality
A beneficial effect experienced by a third party or the society as a whole, resulting from an economic transaction.
Q7: Which of the following substances would you
Q15: Which of the following is a possible
Q25: Describe the similarity and the difference between
Q30: The relative energies (strengths) of the intermolecular
Q85: What is the frequency (<font face="symbol"></font>, in
Q88: Resonance structures indicate that _<br>A)there is more
Q91: Which one of the following diagrams represents
Q125: Which electron-pair geometry has the lowest electron-electron
Q155: Henry's law constant (mol/L · atm) for
Q165: Which of the following types of electromagnetic