Boron nitride is being investigated in frontier research directed at producing novel electronic devices. If you used the following energy-level diagram for the molecular orbitals of boron nitride, BN, what would you predict? These molecular orbitals are formed from the 2s and 2p atomic orbitals. 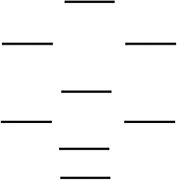 I. Boron nitride is diamagnetic. II. Boron nitride has a bond order of 2.
I. Boron nitride is diamagnetic. II. Boron nitride has a bond order of 2.
III) Boron nitride is paramagnetic.
IV) The bond in BN is stronger than the bond in BN.
Definitions:
International Trade
The exchange of goods, services, and capital between countries, driven by comparative advantages and economies of scale.
Financial Account
A component of the balance of payments that records transactions involving financial assets and liabilities between residents of a country and non-residents.
Oil Imported
The total volume of crude oil and petroleum products that a country acquires from foreign sources to meet its energy and fuel needs.
Dividends
Payments by a corporation of all or part of its profit to its stockholders (the corporate owners).
Q10: What is the orbital designation for an
Q14: Which one of the ionic compounds below
Q40: A coordinate system is defined with orthogonal
Q41: Which of the following is the correct
Q49: Which of the following will have the
Q74: Which of the following materials could be
Q98: CH<sub>2</sub>F<sub>2</sub> has a dipole moment of 1.93
Q114: A saline solution is administered intravenously to
Q148: The mathematical description of an electron as
Q164: What is the molecular geometry around a