A Lewis structure of aspirin without the nonbonding electrons is shown in the figure below. Taking into account the nonbonding electrons, identify the hybridization of the atomic orbitals for the following atoms: O1, O2, and O3. Identify the bond angles around C1, C2, and O3. 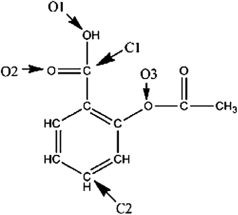
Definitions:
Dissociative Fugue
A rare psychological state where an individual loses awareness of their identity and may unexpectedly travel away from their home or work.
Western Cultures
Societies or nations typically located in the Western hemisphere, characterized by distinct customs, legal systems, and moral values.
Panic Attacks
Short, intense periods during which an individual experiences physiological and cognitive symptoms of anxiety, characterized by intense fear and discomfort.
Dissociative Identity Disorder
A severe form of dissociation, a mental process that produces a lack of connection in a person's thoughts, memory, and sense of identity, formerly known as multiple personality disorder.
Q12: Which one of these samples contains the
Q16: Which liquid, water or ethanol, would you
Q53: Indicate the gas with the lowest density
Q92: Suppose 100.0 mL of a 2.50 mM
Q100: Indicate which aqueous solution has the lowest
Q102: What is the valence electron molecular orbital
Q103: Identify the molecule below that contains a
Q116: In VSEPR theory, molecular geometry is determined
Q148: Which of the following compounds would be
Q156: Which bond angle is the smallest?<br>A)H-C-H in