Multiple Choice
What is the formal charge of each atom (from left to right) , neglecting the hydrogens, in the following resonance structure of CH2N2? 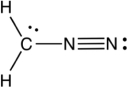
Definitions:
Related Questions
Q12: Which orbital has the highest energy in
Q19: Which one of the following statements is
Q21: Which of the following types of electromagnetic
Q64: Identify the hybridization of the three carbon
Q78: Indicate the ion that does not have
Q85: Chlorofluorocarbons (CFCs or freon) are linked to
Q93: Which of the following molecules or ions
Q96: The freezing point of a 0.060 m
Q120: Calculate the minimum pressure that must be
Q176: According to band theory, when the lower