What wavelength of light is required to cause the ejection of photoelectrons with kinetic energies of 5.0 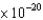 J from a calcium surface ( 4.60
J from a calcium surface ( 4.60 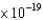 J) ?
J) ?
Definitions:
Defamatory Communication
It refers to the act of making false statements about someone that harm their reputation.
Libel
Written defamation that unjustly harms someone's reputation.
Battery
An intentional and unlawful physical act that results in contact with another person without their consent.
Physical Injury
Harm or damage to the body resulting from an external force, potentially causing pain, disability or death.
Q11: If a chemical reaction causes the temperature
Q25: What angles are present between atoms in
Q33: An orbital's shape is determined by _<br>A)the
Q36: Which transition in a hydrogen atom will
Q46: According to the first law of thermodynamics,
Q51: Carbon dioxide is being used as an
Q78: Indicate the ion that does not have
Q120: In carrying out a titration of a
Q138: Why do the strengths of dispersion interactions
Q156: Which of the following bonds is the