Consider the radial distribution shown below for an electron in an s atomic orbital where r is the distance of the electron from the nucleus. 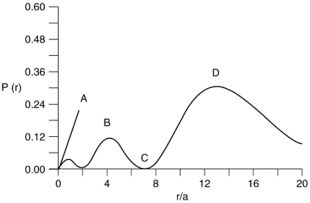 Identify: (1) where the atomic nucleus is located; (2) a radial node; (3) the most probable distance of the electron from the nucleus; and (4) the principle quantum number for this orbital.
Identify: (1) where the atomic nucleus is located; (2) a radial node; (3) the most probable distance of the electron from the nucleus; and (4) the principle quantum number for this orbital.
Definitions:
Reaction
A reaction involves the transformation of one or more substances into different substances through the breaking and forming of chemical bonds.
Exothermic
An exothermic reaction is a chemical reaction that releases heat and energy to its surroundings.
Activation Energy
Activation energy is the minimum quantity of energy which the reacting species must possess in order to undergo a specified reaction.
Flashover
An event in which an entire room or area bursts into flames almost simultaneously, often due to the rapid spread of fire through combustible materials.
Q62: What is the molecular geometry of the
Q66: Which combination of quantum numbers is possible
Q88: Resonance structures indicate that _<br>A)there is more
Q97: How many bonding electrons are assigned to
Q97: What is meant when two or more
Q98: When 2.50 g of sucrose (molar mass
Q111: Write the complete ionic equation for the
Q113: Based on the positions of the atoms
Q116: What is the minimum frequency of a
Q124: In terms of the enthalpy of formation,