You have a summer job in a lead foundry. Your task is to identify how energy efficiency can be improved. You therefore need to know the minimum amount of energy it takes to raise 1 pound of lead (454 g) from room temperature (25 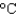 ) to its melting point (327
) to its melting point (327 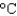 ) and then melt it. The specific heat capacity of lead is 0.159 J/(g
) and then melt it. The specific heat capacity of lead is 0.159 J/(g 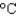 ) , the enthalpy of fusion is 24.7 J/g, and the molar mass is 207 g/mol.
) , the enthalpy of fusion is 24.7 J/g, and the molar mass is 207 g/mol.
Definitions:
Previews and Reviews
A method used in presentations and writings to give a brief introduction or summary before the main content and a recap or evaluation afterwards.
Impersonal Journalism Style
A reporting approach that emphasizes objectivity, factual reporting, and a detached tone, avoiding personal bias or opinion.
Formal Business Report
A structured document that presents information and analysis to assist in business decision-making, often following a standardized format.
Second-Person Pronouns
Pronouns that refer directly to the reader or listener, typically "you," "your," and "yours," used to address the audience directly.
Q18: Ammonia undergoes combustion to produce nitrogen monoxide
Q25: Given the following thermochemical equation detailing the
Q35: Which picture best represents an atomic-level view
Q47: A <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="A O
Q56: For which of the following compounds are
Q69: Use the following information to determine the
Q95: Which prediction regarding the formation of a
Q99: Fool's Gold is the mineral pyrite (FeS<sub>2</sub>).
Q104: Which of these statements about greenhouse gases
Q115: Which one of these samples contains the