Use the following information to determine the enthalpy for the reaction shown below. CS2(l) 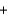 3O2(g)
3O2(g)  CO2(g)
CO2(g) 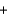 2SO2(g) H ?
2SO2(g) H ?
C(graphite) 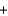 O2(g)
O2(g)  CO2(g) H 393.5 kJ
CO2(g) H 393.5 kJ
S(s) 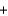 O2(g)
O2(g)  SO2(g) H 296.8 kJ
SO2(g) H 296.8 kJ
C(graphite) 2S(s)  CS2(l) H 87.9 kJ
CS2(l) H 87.9 kJ
Definitions:
School-Related Problems
Issues encountered in the educational setting that can impact a student's learning and social experiences, including academic difficulties, bullying, and lack of resources.
Intimacy
The close, familiar, and usually affectionate or loving personal relationship with another person or group.
Adolescent Friendships
Relationships between teenagers that play a critical role in their emotional and social development, often characterized by deep bonds, emotional support, and identity formation.
Interdependence
A mutual reliance among members of a group or system, where the actions of each individual can affect the whole.
Q2: Thermochemistry is the study of how _
Q4: If 50.0 mL of a 0.10 M
Q6: An element _<br>A)can be separated into its
Q12: In one analysis, 3.01 <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="In
Q20: State Hess's law as it applies to
Q48: Which of the transitions in the hydrogen
Q54: Write Dalton's law of partial pressures in
Q125: Which one of the following statements is
Q132: What is the difference between a strong
Q145: What is the energy (E, in J)