Determine the enthalpy of formation for glucose (C6H12O6) given the following enthalpies of formation (CO2(g) , 393.5 kJ/mol; H2O(l) , 285.8 kJ/mol) and the enthalpy for the reaction shown below. C6H12O6(s) 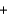 6O2 (g)
6O2 (g)  6CO2(g)
6CO2(g) 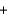 6H2O(l) H
6H2O(l) H  2801 kJ
2801 kJ
Definitions:
Security Interest
A legal claim or right granted over assets as collateral to secure the performance of an obligation, usually repayment of a loan.
Chapter 7
A section of the U.S. Bankruptcy Code that provides for the liquidation of a debtor's assets to pay off creditors.
Good Faith
An honest intent to act without taking an unfair advantage over another person in a transaction.
Chapter 13
A form of U.S. bankruptcy allowing individuals earning regular income to develop a plan to repay all or part of their debts over time.
Q4: The van der Waals equation for real
Q16: Balance the following combustion reaction and report
Q18: Ammonia undergoes combustion to produce nitrogen monoxide
Q26: When are an empirical formula and a
Q28: When a certain acid and a certain
Q78: Identify the incorrect statement(s). A solution _<br>I.
Q79: The elements below are used in fireworks.
Q107: You hold a 50 g sphere of
Q119: How many moles of ammonia are there
Q158: The atomic radius of germanium (Z <img