Determine the enthalpy for the following reaction, given 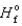 (NH3(g) ) 46.1 kJ/mol,
(NH3(g) ) 46.1 kJ/mol, 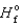 (NO(g) )
(NO(g) ) 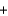 90.3 kJ/mol, and
90.3 kJ/mol, and 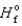 (H2O(g) ) 241.8 kJ/mol. 4NH3(g)
(H2O(g) ) 241.8 kJ/mol. 4NH3(g) 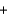 5O2(g)
5O2(g)  4NO(g)
4NO(g) 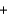 6H2O(g) Hrxn ? kJ
6H2O(g) Hrxn ? kJ
Definitions:
Ford Motor Company
An American multinational automaker founded by Henry Ford in 1903, known for revolutionizing the automobile industry with assembly line production.
Hull House
An iconic settlement house founded by Jane Addams and Ellen Gates Starr in Chicago in 1889, aimed at improving the lives of the community's immigrant population.
Americanize
The process of assimilating or causing someone to assimilate into American culture and norms.
Eugenics
A set of beliefs and practices aimed at improving the genetic quality of the human population, historically associated with morally problematic policies like forced sterilization.
Q3: Which one of the following is not
Q11: How much energy is required to ionize
Q33: Radium often is found in uranium ores
Q34: Which subshell contains six total electrons?<br>A) <img
Q54: Write Dalton's law of partial pressures in
Q92: A shell consists of all _<br>A)electrons with
Q118: Controlling the concentration of ammonia is
Q120: What color will a blue object appear
Q139: Which statement is not correct? In atomic
Q157: An unknown gas held in a 25.0