Determine the enthalpy for the following reaction, given 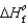 (NH3(g)) 46.1 kJ/mol,
(NH3(g)) 46.1 kJ/mol, 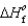 (NO(g))
(NO(g)) 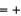 90.3 kJ/mol, and
90.3 kJ/mol, and 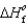 (H2O(g))
(H2O(g))  241.8 kJ/mol.
241.8 kJ/mol.
4NH3(g)  5O2(g)
5O2(g)  4NO(g)
4NO(g) 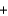 6H2O(g) Hrxn
6H2O(g) Hrxn  ? kJ
? kJ
Definitions:
Cohort Effect
Differences between age groups that arise not necessarily from aging but from the unique social and environmental influences affecting those groups during their developmental periods.
Time Lag
The delay between the initiation of an action or process and its effect or outcome.
Standardized Test
A test in which an individual’s score is compared to the scores of a group of similar individuals.
Correlational Coefficient
A statistical measure that indicates the extent to which two or more variables fluctuate together.
Q35: Even though lead is toxic, lead compounds
Q39: Phthalocyanine is a large molecule used in
Q51: Assuming that the distances between the two
Q67: Which set of gases is listed from
Q70: The temperature of a 10.0 g sample
Q73: How many grams of solid magnesium chloride,
Q76: Which one of the following reaction equations
Q117: What is the correct symbol for a
Q130: Which one of the following is an
Q159: What color will a red object appear