The energy content of a Big Mac is 540 Cal. How much water can be heated from 20 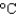 to 90
to 90 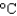 by this amount of energy? (cP(water) 1.00 g/mL, cs(water) 4.18 J/(g
by this amount of energy? (cP(water) 1.00 g/mL, cs(water) 4.18 J/(g 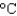 ) , 1 Cal 1,000 cal 4.184 kJ)
) , 1 Cal 1,000 cal 4.184 kJ)
Definitions:
Product Cost
The total expense incurred to produce a product, including direct materials, direct labor, and overhead costs.
Incremental Loss
The additional loss incurred from producing one extra unit of a product or service beyond the break-even point.
Special Order
An order for a product that is made to a customer's specifications, differing from regular stock products.
Capacity
The maximum level of output that a company can sustain to produce in a given period under normal circumstances.
Q14: Which statement about the quantum numbers that
Q27: Explain what is meant by the term
Q30: List three reasons why the actual yield
Q34: Which subshell contains six total electrons?<br>A) <img
Q52: According to Coulomb's law, which ionic compound
Q58: What mass of phosphoric acid (H<sub>3</sub>PO<sub>4</sub>, 98.0
Q88: In which compound does chlorine have an
Q112: Combustion analysis of an organic compound to
Q125: Determine the wavelength of the line in
Q137: Give an example of an alkali metal.