You have a summer job in a lead foundry. Your task is to identify how energy efficiency can be improved. You therefore need to know the minimum amount of energy it takes to raise 1 pound of lead (454 g) from room temperature (25 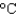 ) to its melting point (327
) to its melting point (327 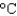 ) and then melt it. The specific heat capacity of lead is 0.159 J/(g
) and then melt it. The specific heat capacity of lead is 0.159 J/(g 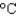 ) , the enthalpy of fusion is 24.7 J/g, and the molar mass is 207 g/mol.
) , the enthalpy of fusion is 24.7 J/g, and the molar mass is 207 g/mol.
Definitions:
ENTER Key
A keyboard key used to submit information to a program or trigger a command.
Web Address
A unique string of characters that identifies a specific resource on the internet, typically leading to a webpage.
Complimentary Close
A complimentary close is a polite phrase used to end a letter or email, such as "Sincerely" or "Best regards."
Business Letter
A formal document used for communication between companies, or between a company and its clients, suppliers, or other external parties, adhering to a standard format and tone.
Q5: Which statement A-D about energy units is
Q15: What is the temperature of a sample
Q34: Energy that an object has by virtue
Q44: Which polyatomic ion is not labeled correctly?<br>A)NH<sub>4</sub><sup>+</sup>ammonium<br>B)ClO<sub>4</sub><sup>-</sup>
Q47: What is the kinetic energy of the
Q49: A sample of water (H<sub>2</sub>O) contains 1.81
Q95: Which one of the molecules below has
Q99: If there are 0.505 g of NaCl
Q121: Which one of the following mixtures is
Q145: When sulfur-containing fuels are burned, sulfur trioxide