Use the following information to determine the standard enthalpy change when 1 mol of PbO(s) is formed from lead metal and oxygen gas. PbO(s) 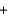 C(graphite)
C(graphite)  Pb(s)
Pb(s) 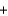 CO(g) H
CO(g) H  107 kJ
107 kJ
2C(graphite) 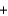 O2(g)
O2(g)  2CO(g) H 222 kJ
2CO(g) H 222 kJ
Definitions:
Chimpanzee Hunting
The practice whereby chimpanzees, a species of great ape, organize and collaborate in hunting smaller primates or other animals for food, demonstrating complex behavior and social cooperation.
Common Heritage
Principles or assets that are not only shared by and beneficial to all members of a community or humanity but also preserved for future generations.
Federal Facility
A building or installation owned or leased by the federal government of a country, often for governmental use.
Biomedical Research
The field of study focused on the investigation of biological processes and diseases with the aim of developing new treatments and understanding human health.
Q1: The following diagrams illustrate the flow of
Q11: If a chemical reaction causes the temperature
Q16: Balance the following combustion reaction and report
Q32: In a mixture of gases, the gas
Q41: During a(n) _ process, energy is transferred
Q42: Write the equation that corresponds to the
Q82: The diffusion rate of H<sub>2</sub> in a
Q105: A <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB3835/.jpg" alt="A Mn<sup>2+</sup>
Q143: Ca is the symbol for _<br>A)cesium.<br>B)cobalt.<br>C)cadmium.<br>D)calcium.<br>E)cerium.
Q144: What is the formula for calcium nitride?<br>A)CaN<br>B)Ca<sub>2</sub>N<sub>3</sub><br>C)Ca<sub>2</sub>N<br>D)Ca<sub>3</sub>N<sub>2</sub><br>E)CaN<sub>2</sub>