Use the following information to determine the enthalpy for the reaction shown below. CS2(l) 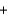 3O2(g)
3O2(g)  CO2(g)
CO2(g) 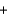 2SO2(g) H ?
2SO2(g) H ?
C(graphite) 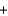 O2(g)
O2(g)  CO2(g) H 393.5 kJ
CO2(g) H 393.5 kJ
S(s) 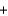 O2(g)
O2(g)  SO2(g) H 296.8 kJ
SO2(g) H 296.8 kJ
C(graphite) 2S(s)  CS2(l) H 87.9 kJ
CS2(l) H 87.9 kJ
Definitions:
Underproduction
A situation where the quantity produced of a good or service is less than the socially optimal level, often leading to market failure.
Market Price
The existing cost for acquiring or offloading an asset or service in the marketplace.
Consumer Surplus
The difference between the maximum price consumers are willing to pay and the actual price they pay.
Deadweight Loss
A loss of economic efficiency that occurs when the equilibrium for a good or service is not achieved or is not achievable due to market distortions like taxes or subsidies.
Q4: Cesium is an example of _<br>A)an alkali
Q16: Write the net ionic equation for the
Q39: Phthalocyanine is a large molecule used in
Q49: A sample of water (H<sub>2</sub>O) contains 1.81
Q71: Which particle-level diagram is the best representation
Q83: In an experiment, 74.3 g of metallic
Q89: In which of the following molecules does
Q108: Carbon monoxide is used in refining iron
Q127: Mesitylene is a liquid hydrocarbon. If 0.115
Q134: A barometer measures a pressure of 745