Determine the standard enthalpy of formation for NO given the following information about the formation of NO2 under standard conditions, and 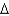
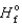 (NO2)
(NO2) 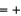 33.2 kJ/mol. 2NO(g)
33.2 kJ/mol. 2NO(g) 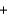 O2(g)
O2(g)  2NO2(g) Hrxn
2NO2(g) Hrxn  114.2 kJ
114.2 kJ
Definitions:
Crowdsourcing
The method of acquiring ideas, services, or content by requesting contributions from a broad group of individuals, particularly from an online community.
Wikipedia
A free, multilingual online encyclopedia written and maintained by a community of volunteer contributors through a model of open collaboration.
Evidence-based Medicine
A clinical practice approach that emphasizes the use of current best evidence in making decisions about the care of individual patients.
Moneyball
refers to the strategy of using advanced statistical analysis and empirical data to make decisions, especially in the context of sports team management.
Q8: Which one of the following molecules violates
Q37: Assuming that the distance between ions remains
Q80: Hydrogen peroxide decomposes to produce water
Q84: Which set of gases is listed from
Q99: Fool's Gold is the mineral pyrite (FeS<sub>2</sub>).
Q113: How many unpaired electrons does the nitride
Q121: Which one of the following mixtures is
Q144: A balloon is filled with 3.00 L
Q149: Which monatomic ion most likely does not
Q166: Which of the following will lead to