Short Answer
Given the following thermochemical equation detailing the combustion of methane
CH4(g) 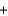 2O2(g)
2O2(g)  CO2(g)
CO2(g) 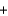 2H2O(g) Hrxn 802 kJ/mol CH4
2H2O(g) Hrxn 802 kJ/mol CH4
determine the amount of energy released when 25 g of methane undergoes combustion.
Definitions:
Related Questions
Q12: Why is an aqueous solution of table
Q18: What is the change in internal energy
Q31: Which set of gases is listed from
Q40: The oxidation number of carbon in carbon
Q52: Which one of the following is a
Q99: Copper forms a cation with a charge
Q104: The composition of Earth's atmosphere by volume
Q109: Assuming that the distances between the two
Q127: The studies of light emitted by hot
Q144: Down a column in the periodic table,